What are the possible isomers for #H_3C - CH_2 - O - CH_2 - CH_2 - CH_3#? In which class do each of these isomers fall into (eg. chain, positional, functional isomerism)?
1 Answer
I count 14 isomers.
Explanation:
Let's start with the alcohols.
Five carbons in a row
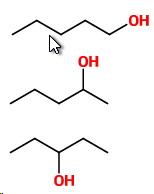
Three isomers are
1. Pentan-1-ol
2. Pentan-2-ol
3. Pentan-3-ol
Four carbons with a methyl side-chain
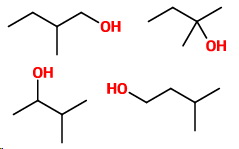
Four more isomers are
4. 2-Methylbutan-1-ol
5. 2-Methylbutan-2-ol
6. 3-Methylbutan-2-ol
7. 3-Methylbutan-1-ol
Three carbons with two methyl groups
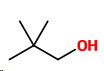
Only one more isomer
8. 2-2-Dimethylpropan-1-ol
Now we do the ethers
Start by inserting an oxygen into a five-carbon chain.
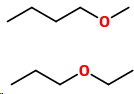
This gives two more isomers
9. 1-Methoxybutane
10. 1-Ethoxypropane
Repeat with four carbons and a methyl side-chain
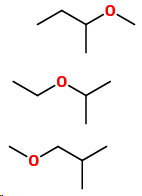
Three more isomers
11. 2-Methoxybutane
12. 2-Ethoxypropane
13. 1-Methoxy-2-methylpropane
And finally, a three-carbon chain with two methyl groups.
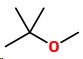
The final isomer
14. 2-Methoxy-2-methylpropane
And we now have all the constitutional isomers (I hope!).
"We leave it as an exercise for the student" to identify the chain, positional, and functional isomers.

