What are the possible values of n and ml for an electron in a 5d orbital ? A) n = 5 and ml = -2, -1, 0, +1, or +2 B) n = 1, 2, 3, 4, or 5 and ml = 2 C) n = 5 and ml = 2 D) n = 1, 2, 3, 4, or 5 and ml = -2, -1, 0, +1, or +2
1 Answer
The answer is A).
The principal quantum number, or
The angular momentum quantum number, or
The value of
The accepted values for
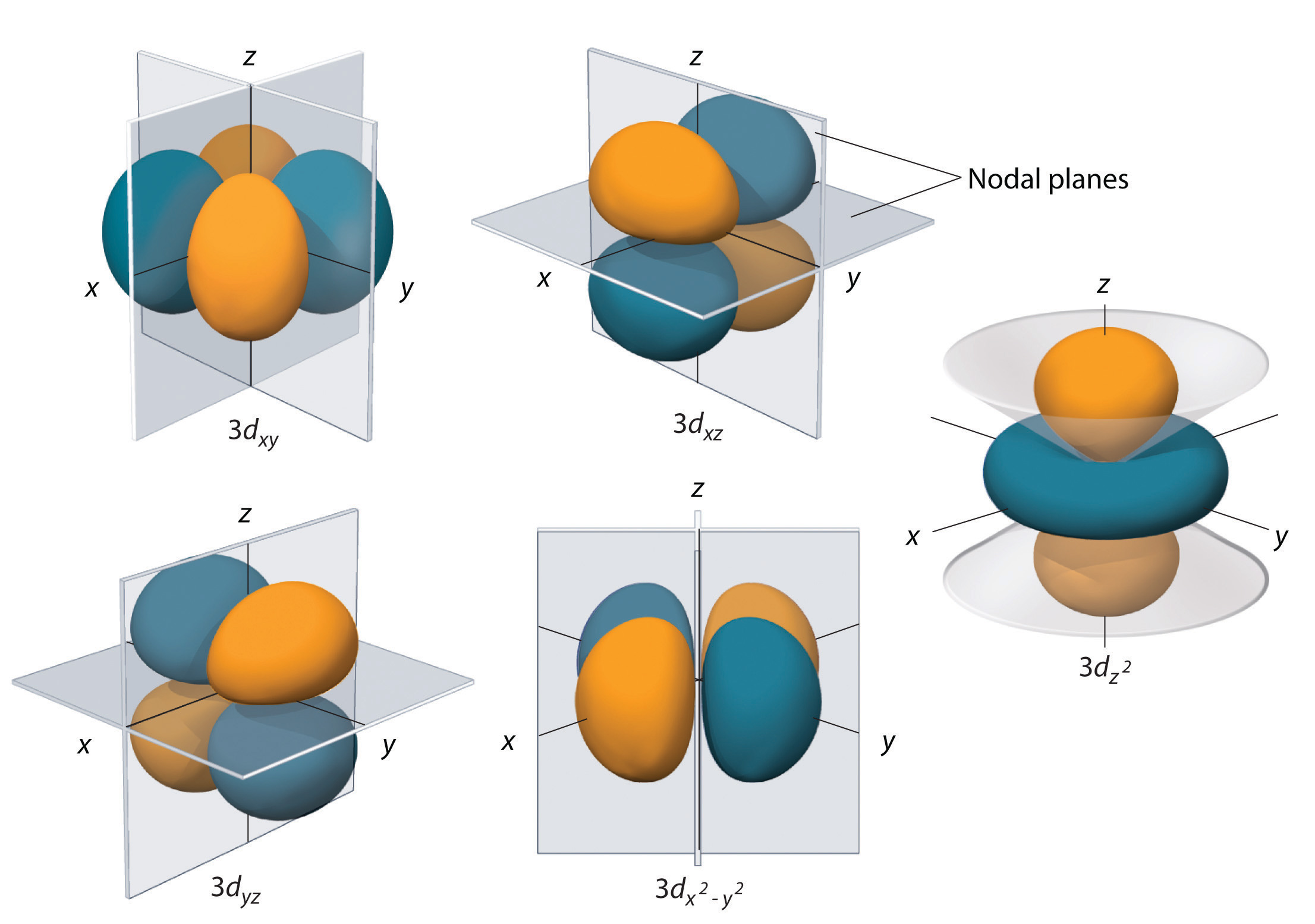
As an example, here's how these orbitals would look for the 3d subshell