What aromatic compounds follow Hückel's rule?
1 Answer
All aromatic compounds follow Hückel's rule.
Explanation:
Hückel's rule states that a planar, cyclic, conjugated molecule is aromatic if it contains 4
See Can I determine aromaticity by Hückel's rule?
Compounds with
The most common examples have
a. Benzene and its derivatives
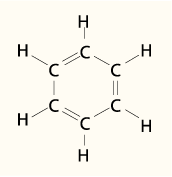 www.worldofmolecules.com
www.worldofmolecules.com
b. Six-membered heterocyclics like pyridine and pyrimidine
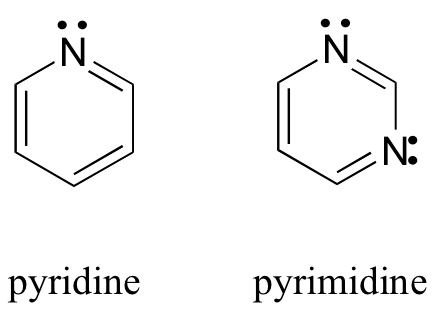 chemwiki.ucdavis.edu
chemwiki.ucdavis.edu
c. Five membered heterocyclics like pyrrole, furan, and thiophene
 intranet.tdmu.edu.ua
intranet.tdmu.edu.ua
d. Five-membered anions like cyclopentadienide ion
 image.tutorvista.com
image.tutorvista.com
e. Seven-membered cations like tropylium ion
 classconnection.s3.amazonaws.com
classconnection.s3.amazonaws.com
Compounds with
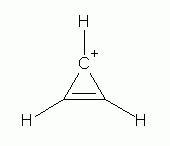 3.bp.blogspot.com
3.bp.blogspot.com
cyclopropenyl cation
Compounds with
These include naphthalene and azulene.
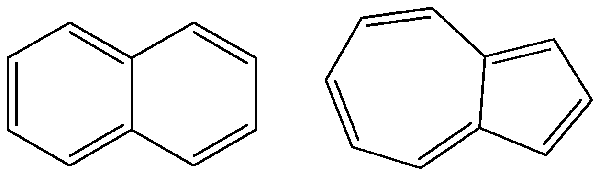 gaussian.com
gaussian.com
Compounds with
These include the annulenes.
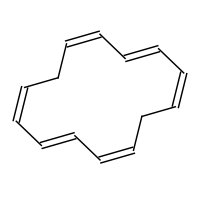 www.click2drug.org
www.click2drug.org
[14]-annulene (
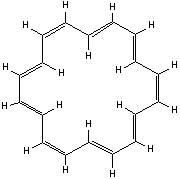 www.uea.ac.uk
www.uea.ac.uk
[18]-annulene (

