What atomic transition occurs in the atoms of hydrogen gas in the spiral arms of our Galaxy to produce the 21-cm radio emission?
1 Answer
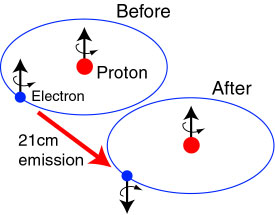
Because of the magnetic dipole interaction between the electron and nucleus the ground state energy level of an electron is split into two. The 21 cm radiation is a result of electronic transitions across these states.
Explanation:
If we assume the electronic interaction with the atomic nucleus to be purely electrostatic then we have only one ground state energy level ( called the 1s state in spectroscopic notation).
But electrons and protons (hydrogen nuclei) seem to have intrinsic angular momenta (called spin). Any charged particle with intrinsic angular momentum will have a magnetic moment. It is as though electron and proton are two magnetic with their own magnetic moments. The magnetic interaction between these two lead to the splitting of the ground state energy level into two separate energy energy levels. This splitting of ground state energy level is called the hyperfine structure.
When the electron's spin magnetic moment is aligned parallel to the spin magnetic moment of the nucleus it is a state of higher energy. When the electron spin magnetic moment is aligned anti-parallel to the spin magnetic moment of nucleus its is a state of lower energy.
The energy difference between these states is:
Transition across these states involve photon frequencies of