What causes geometric isomers?
1 Answer
The potential of certain molecules to give a number of distinct geometries..........
Explanation:
Geometric isomers are molecules with the same chemical formula, and the SAME CONNECTIVITY that have different geometries.
The best and most simple example of geometric isomerism occurs for
Because the methyl groups can be on the SAME or DIFFERENT sides of the olefinic bond, the structure can generate 2 geometric isomers:
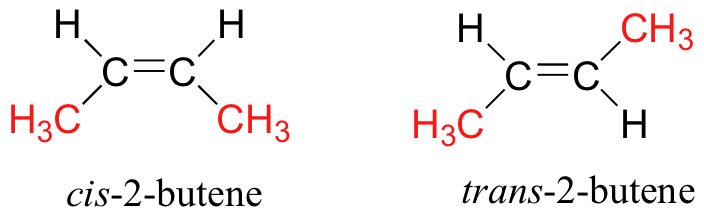
For both isomers, the

