What decides the color of a compound ? I want a brief explanation.
2 Answers
The number of valence electrons.
Explanation:
The number of valence electrons which are present in the outermost orbit of a compound decide the colour of light. These electrons absorb a certain portion of visible light spectrum and produce a colour that is complementary to the colour of the light absorbed.
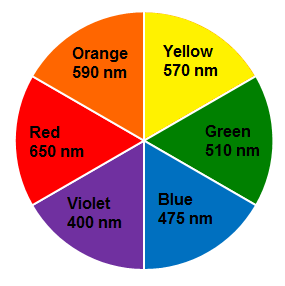
Example: If a compound absorbs violet colour, it shows green-yellow colouration (complementary to violet).
The difference between electronic energy levels determines the colour of a compound.
Explanation:
A compound can absorb energy when the wavelength of electromagnetic radiation is enough to raise it to a higher level (an excited state).

If the energy difference is in 400 nm to 800 nm region, the compound absorbs visible light.
However, the eye does not see the colour absorbed.
Instead, it sees the complementary colour caused by the removal of the absorbed wavelengths.

Thus, a compound that absorbs at 570 nm appears as violet to the eye.