What do metals typically do when they become ions? What about nonmetals?
1 Answer
Feb 10, 2017
Metal atoms lose one or more electrons when they become ions. Nonmetal atoms gain one or more electron when they become ions.
Explanation:
Atoms form ions so that they will have a full valence shell, which is 8 electrons (2 for hydrogen).
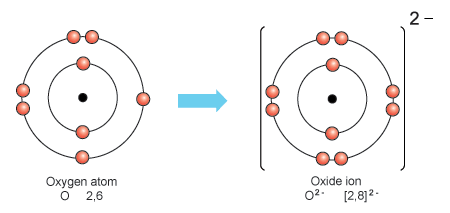
Sodium, a metal, loses its single valence electron so that the next lower energy level becomes its valence shell with 8 electrons. Losing the single electron gives the sodium ion a
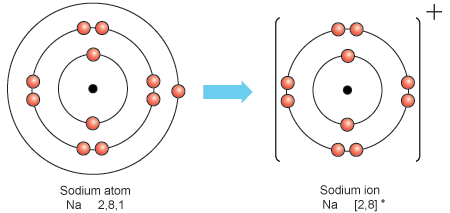
Oxygen, a nonmetal, has 6 electrons in its valence shell, so it gains 2 electrons and forms a