What element is in period 4 and has 4 number shells and 3 valence electrons?
Can't find it anywhere
Can't find it anywhere
2 Answers
Gallium (Ga)
Explanation:
Gallium is in period 4 on the periodic table and has 31 electrons:
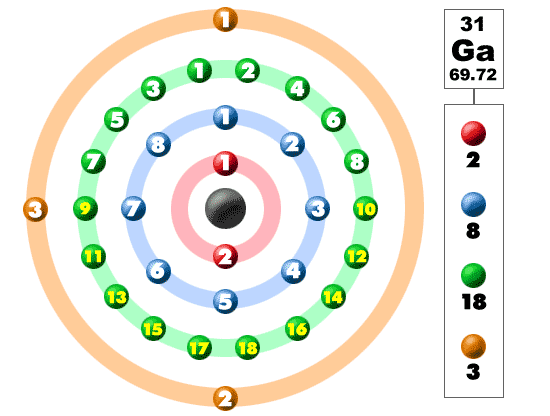
Sc number 21 Scandium
Explanation:
Scandium atomic number 21 is in the fourth period.
The electron configuration of Scandium is
Scandium has three different kinds of subshells
s, p and d .
Scandium has four different main electron shells
1st , 2st , 3rd and 4th .
Scandium has 3 valance electrons
2 4s electrons
1 3d electron
for a total of 3 valance electrons.


