What functional groups are present in acetaminophen?
1 Answer
The functional groups in acetaminophen are hydroxyl, aromatic ring, and amide.
Explanation:
A functional group is a specific group of atoms within a molecule that gives rise to the characteristic chemical reactions of the molecule.
The structure of acetaminophen is
The group at the top of the molecule is a hydroxyl group. It is tempting to call it an alcohol group.
But an
The six-membered ring is an aromatic ring.
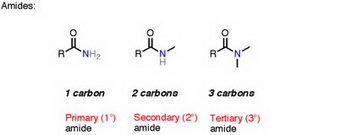
The group at the bottom of the molecule is a monosubstituted or secondary amide.
The general formula for an amide is