What happens as you decrease the temperature of a solution?
1 Answer
It depends on what the solution is made of.
Explanation:
Solutions contain a solute and a solvent. One example of a solution is the carbon dioxide gas which is dissolved in seltzer water. In this case the
One reason this is important is that some species of fish require higher oxygen levels and need to live in lakes with colder average temperatures which will have higher levels of dissolved oxygen. An example of this are lake trout, which thrive in the colder waters of the Great Lakes.

A different example of a solution is when a solid dissolved in a liquid. Sugar (solute) dissolving in water (solvent) is an example of this type of solution. Solids typically become more soluble in liquids as the temperature goes up, so cooling the liquid will cause solute to come out of solution. This will lead to crystallization.
A solubility curve shows how the solubility of different solutes changes with changing solvent temperature. Notice how gases such as
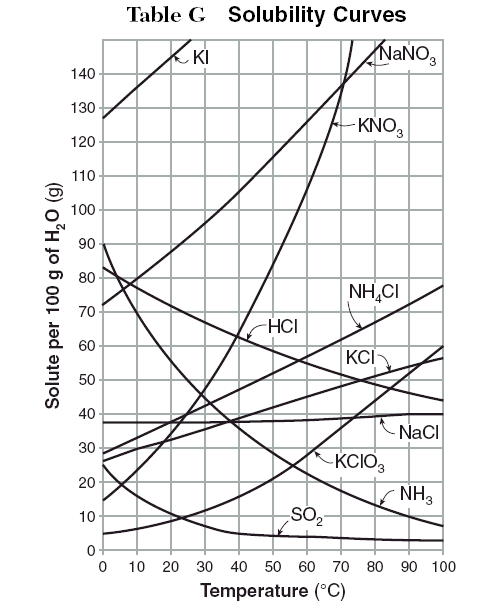
Hope this helps!

