What happens at the cathode in an electrolytic cell?
1 Answer
Mar 12, 2018
At the cathode, reduction takes place.
Explanation:
At the cathode in an electrolytic cell, ions in the surrounding solution are reduced into atoms, which precipitate or plate out on to the solid cathode.
For example, take a look at the following cell:
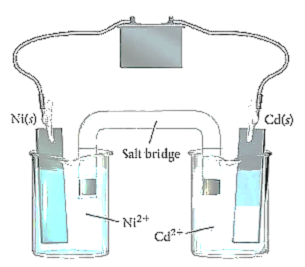 https://www.clutchprep.com/chemistry/practice-problems/8969/consider-the-electrolytic-cell-a-label-the-anode-and-the-cathode-and-indicate-th
https://www.clutchprep.com/chemistry/practice-problems/8969/consider-the-electrolytic-cell-a-label-the-anode-and-the-cathode-and-indicate-th
Here, Cadmium is reduced, and Nickel is oxidized. The following are the two half reactions:
Oxidation:
Reduction:
The anode is where oxidation takes place, and the cathode is where reduction takes place. So in this example, the anode is the solid Nickel electrode, and the cathode is the solid Cadmium electrode.

