What happens on a spectrum when two H's are equivalent?
1 Answer
Sep 16, 2015
When two hydrogen atoms are equivalent, their signals coalesce to give a single peak with twice the area.
Explanation:
If the two hydrogens are non-equivalent but close enough to split each other, you get an AB pattern, a doublet of doublets.
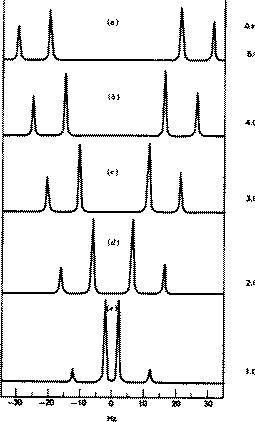
As they become more nearly equivalent, the inner peaks increase in intensity and move closer to each other.
At the same time, the outer peaks decrease in intensity.
At the extreme, when the two hydrogen atoms are equivalent, the inner peaks coalesce to a single peak in the centre, and the intensity of the outer peaks decreases to zero.

