Why are all hydrogens in aromatic rings chemically the same?
1 Answer
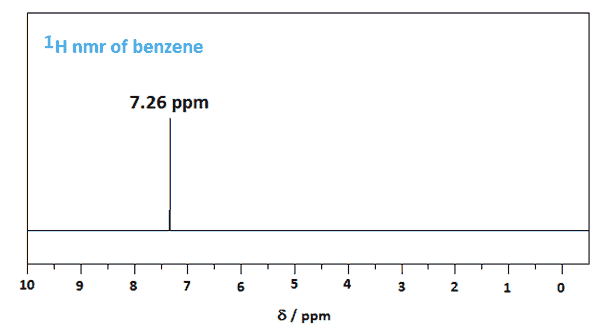
All hydrogens in benzene are chemically the same, but in most aromatic compounds they are not.
You can test for chemical equivalence by mentally replacing each H in turn with another atom (say, Cl).
In benzene, this gives only one compound: chlorobenzene.
So all the H atoms in benzene are chemically equivalent, and benzene gives only a single NMR signal at 7.26 ppm.
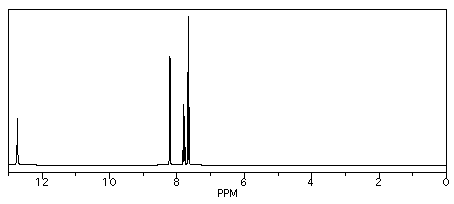
If you carry out the same procedure with benzoic acid, though, you get three different products: 2-, 3-, and 4-chlorobenzoic acid.
So, benzoic acid has three different sets of chemically equivalent hydrogens.
It shows three aromatic peaks near 8 ppm.