What important characteristic do the alkali metals alkaline earth metals have in common?
1 Answer
Atoms from both groups form cations of limited oxidation states as opposed to transition metals that form ions of more than one possible charge .
- Alkali metals form ions of charge
#+1# whereas - Alkaline earth metals form ions of charge
#+2#
Explanation:
Most alkali and alkaline-earth elements have low electronegativity and tend to form cations in chemical processes. Magnitudes of consecutive ionization energies of a particular atom offer insights into the various possibilities of its ion formations.
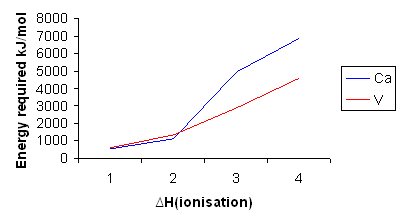
For example, it takes no more than
Alkali and alkaline-earth cations are energetically stable with an empty valence shell. Atoms of alkali and alkaline-earth elements achieve octets after losing one (alkali) or two (alkaline-earth) electrons such that they minimize the potential energy of the system. Ions of charge

