What is a combination of four quantum numbers that could be assigned to an electron occupying a 5p orbital?
1 Answer
Here's what I got.
Explanation:
As you know, you need four quantum numbers to describe the exact location of an electron in a atom and its spin.
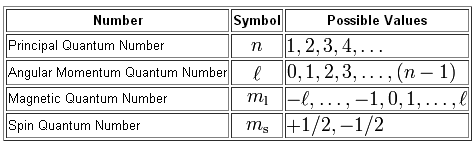
The principal quantum number,
#n = 5 -># the electron is located on the fifth energy level
The angular momentum quantum number,
#l = 0, 1, 2, 3, 4#
Now, as its name suggests, a 5p-orbital will be located in a p-subshell, which is characterized by
#l = 1 -># the electron is located in the 5p-subshell
The magnetic quantum number,
This means that you have three possibilities here, since the exact orbital is not specified by the problem
#m_l = {-1, 0, 1} -># the electron can reside in any of the three 5p-orbitals
Finally, the spin quantum number,
This means that an electron residing in a 5p-orbital can have
#m_s = {+1/2, -1/2} -># either spin-up or spin-down
So, one set of quantum numbers that can describe an electron located in a 5p-orbital is
#n=5, l=1, m_l = -1, m_s = +1/2#
This electron is located on the fifth energy level, in the 5p-subshell, in the
There are a total of six sets of quantum numbers that can be used here.

