What is a compound?
1 Answer
A compound is a pure substance that is composed of two or more elements.
Explanation:
A compound is a pure substance that is composed of two or more elements. Some compounds are formed by covalent bonding, in which valence electrons are shared between atoms; and some compounds are formed by ionic bonding, in which the complete transfer of one or more electrons from one atom to another occurs.
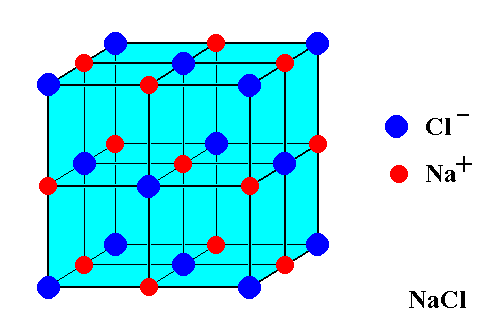
The smallest particle of a covalent compound is called a molecule. Because ionic compounds form crystal lattices, they do not exist as molecules. Instead, the smallest whole number ratio that exists between the cations and anions is called a formula unit.
All compounds have a definite composition. For example, a compound of hydrogen and oxygen that forms water has the formula
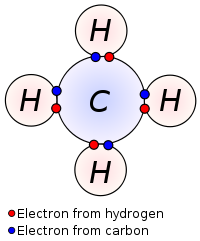
The diagram below represents a molecule of methane gas
The diagram below shows part of a crystal lattice of sodium chloride