What is a conjugate acid and base pair?
1 Answer
Conjugate acid-base pair are compounds which differ by
Explanation:
Here's are two examples of conjugate acid-base pair.
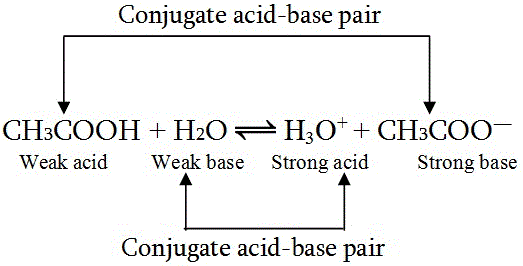
The concept of conjugate acid-base pair is related to Bronsted-Lowry acid-base theory and according to this theory, acid is a proton
Let's focus on the first example,
The product that is formed (
- acid:
#CH_3COOH# - conjugate base: (
#CH_3COO^-# )
You might be wondering, why is
For the second example,
In this case,
- base:
#H_2O# - conjugate acid:
#H_3O^+#
In short,
- conjugate base is formed when an acid donates a proton.
- conjugate acid is formed when a base accepts a proton.

