What is a double covalent bond?
1 Answer
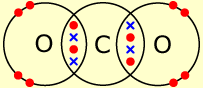
A double covalent bond is one in which two atoms share two pairs of electrons, rather than one.
Explanation:
One example is the molecule carbon dioxide. Its molecular formula is
The diagram below shows the structure of a carbon dioxide molecule. The two red oxygen electrons and two blue carbon electrons in the overlapping regions represent the two double bonds.