What is a first order half life graph?
1 Answer
Jun 24, 2018
Well, first-order half-lives are constants with respect to reactant concentration...
#t_(1//2) = (ln2)/(k)# where
#k# is the first-order rate constant in#"s"^(-1)# ...
So, presumably, you mean, a regular concentration vs. time graph on which the half-life is marked...
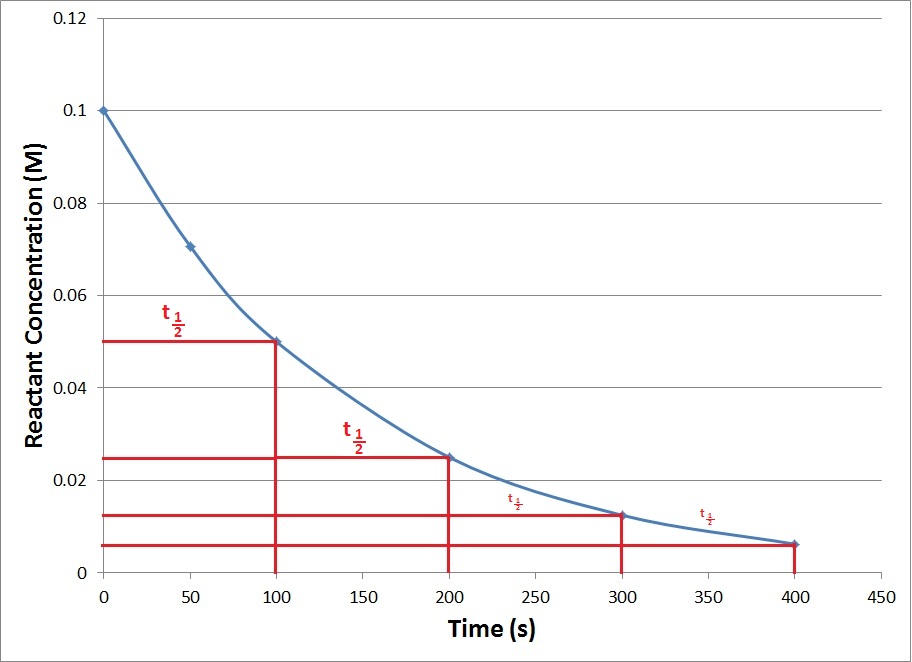
Apparently, this reactant has a half-life of

