What are #S_N1, S_N2, E1 and E2# reactions? How to identify which reaction is occurring in any given reaction.
1 Answer
You should consider several factors:
- Solvent (may or may not deactivate nucleophile)
- Sterics (of substrate or nucleophile; more sterics = less second-order reactions, and vice versa)
- Reagent pKas (high pKas allow elimination as a possible side mechanism)
- Temperature (high temperatures favor elimination)
- Leaving group propensity (great leaving groups favor first-order reactions, such as
#E1# or#S_N1# )
This table summarizes what we'll look at.
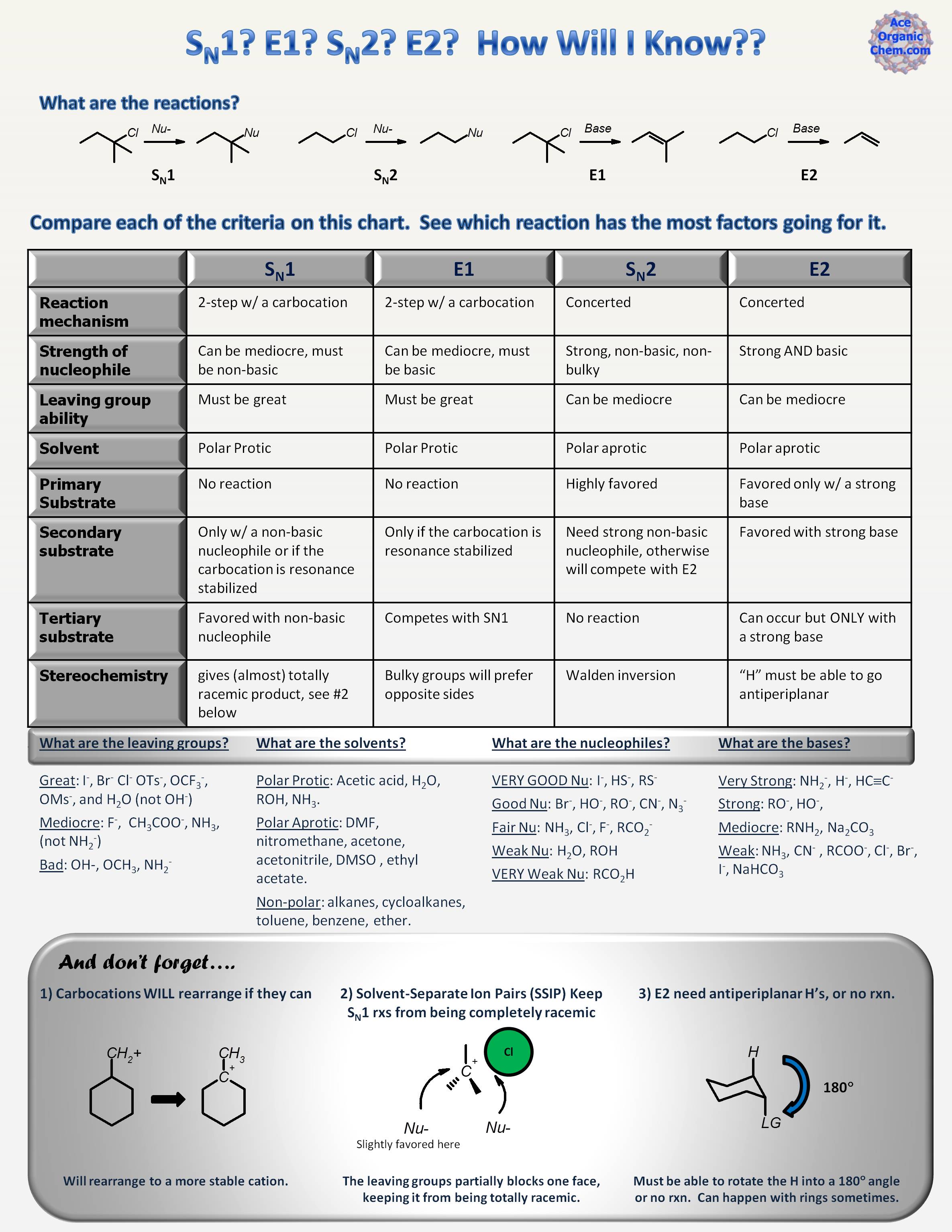
The following examples assume a great leaving group.
SN2 REACTIONS
As a random example, consider a general primary alkyl halide reacting with
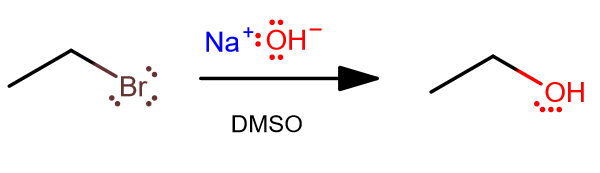
In DMSO, which is a polar aprotic solvent (has no protons, and can dissolve both
That means in this context,
Furthermore, the alkyl halide is not sterically-hindered. That is, it is easy to attack it from behind, because there is no bulkiness around the
Therefore, this situation would be mostly
- the nucleophile is strong
- the primary alkyl halide is sterically unhindered
allowing backside-attack that is typical to
#S_N2# reactions.
This creates a stereochemical inversion.
SN1 REACTIONS
Now modify our example, and use a protic solvent instead, like ethanol. We can still use
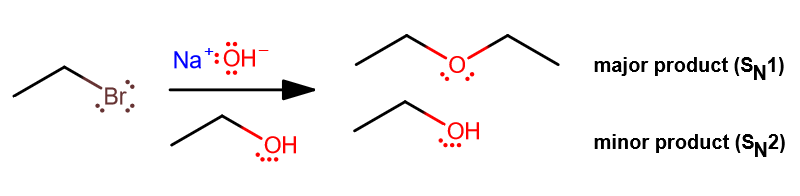
Since
- The major product forms from
#S_N1# instead (unimolecular nucleophilic substitution), since#"OH"^(-)# has become#"H"_2"O"# , which is in general a weak nucleophile. - Some minor side products form when remaining
#"EtOH"# coordinates with any leftover alkyl halide, or when the formed#"EtO"^(-)# reacts with the leftover alkyl halide via#S_N2# .
It's not entirely clear what the product mixture will give us in terms of percentages, but it will not be just
Furthermore, if the alkyl halide happens to be secondary or tertiary, you will get some carbocation intermediate. That would also facilitate
E2 REACTIONS
Now if we modify the
At higher temperatures, elimination is favored over substitution.
Furthermore, the greater sterics on the nucleophile make it a poor nucleophile, but since the alkyl groups are electron-donating, tert-butoxide is still a great base:
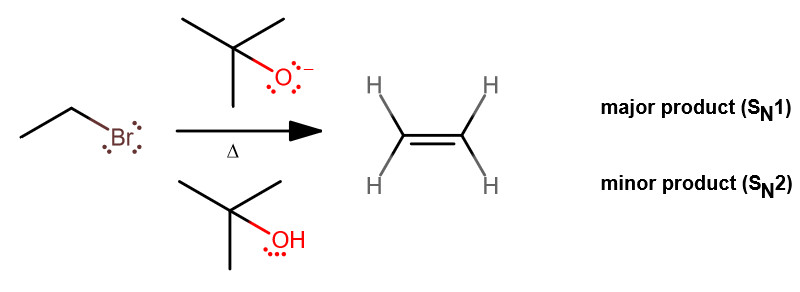
This bimolecular elimination reaction removes one proton and one leaving group (
Most
E1 REACTIONS
This more often occurs when the alkyl halide is also sterically-hindered, but it also somewhat occurs even when
Like

