What is so special about the chair conformation?
1 Answer
The chair conformation is special because it is the most stable arrangement of atoms in the cyclohexane ring.
Explanation:
We tend to think of cyclohexane as a planar ring, as in the diagram below.
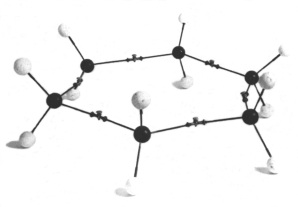
(from www.hyle.org)
But this would be a high-energy structure. The bond angles would be 120° instead of 109.5°, and all the H atoms would be eclipsed.
Some of the angle strain can be relieved if one of the C atoms is lifted out of the ring to form a half-chair.
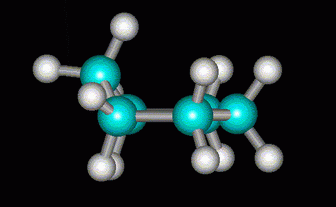
(from course1.winona.edu)
More strain would be relieved if the C atom at the other end were also lifted to form a boat.
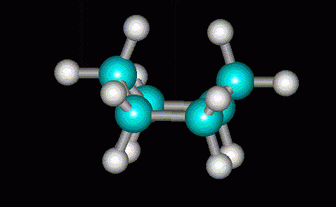
(from course1.winona.edu)
But this introduces severe steric hindrance between two of the H atoms, and the H atoms on the sides are all eclipsed.
If the C atom is bent the other way, we get the chair form of cyclohexane.
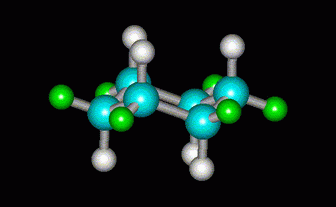
(from course1.winona.edu)
There is no angle strain, and the H atoms are all staggered.
The chair is the most stable form of cyclohexane.
There are actually two forms of the cyclohexane chair.

(from classes.yale.edu)
At room temperature, they interconvert about 100 000 times per second.

