What is the balanced chemical equation for this reaction: Gaseous chlorine reacts with an aqueous solution of potassium bromide to form liquid bromine and an aqueous solution of potassium chloride?
1 Answer
May 15, 2018
Explanation:
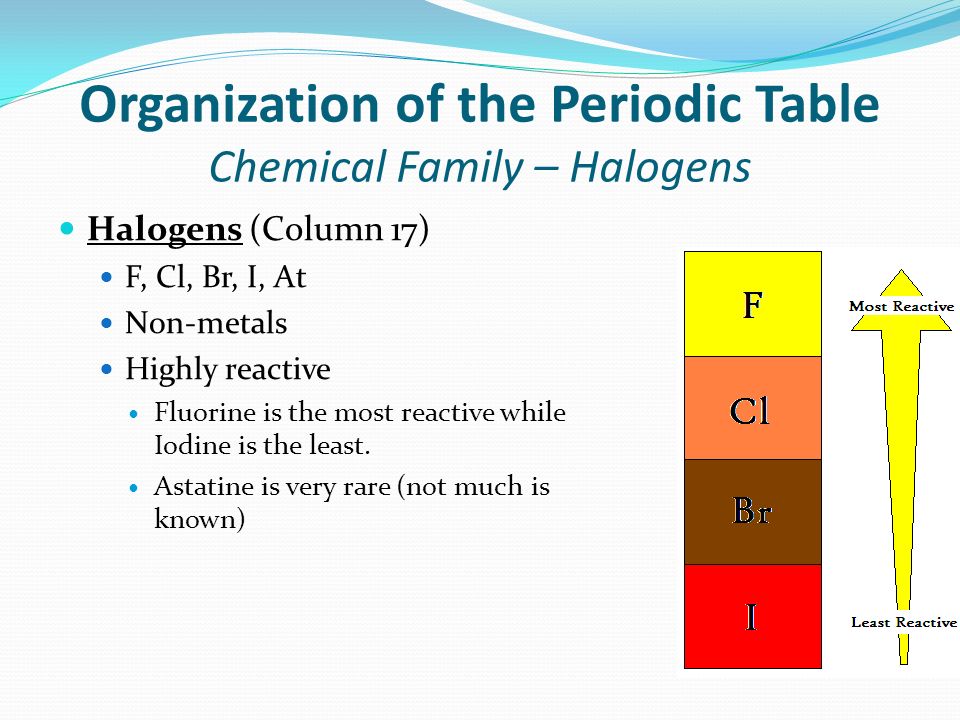
This is a single replacement reaction in which a more reactive halogen (group 17/VIIA) replaces a less reactive halogen in a compound.
The reactivity of the halogens relative to one another decreases down the group. Since chlorine is above bromine, it is more reactive and will replace the bromine in the potassium bromide compound.
However, the reverse reaction would not occur, because bromine is less reactive than chlorine, so it would not be able to replace the chlorine in the potassium chloride compound.