What is the "Carbonic acid-bicarbonate" buffer system?
1 Answer
It's a buffer system that consists of a weak acid and its conjugate base.
Explanation:
The carbonic acid - bicarbonate buffer system consists of carbonic acid, a weak acid, and the bicarbonate anion, its conjugate base.
The important thing to realize here is that carbonic acid,
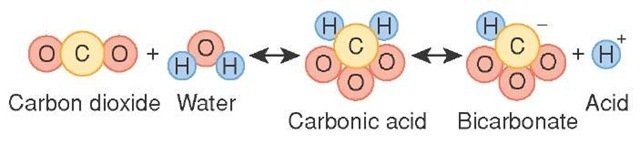
After carbon dioxide is dissolved, it combines with the water molecules to form carbonic acid according to the following equilibrium reaction
Being a weak acid, carbonic acid can then go on and donate its protons in two steps to form the bicarbonate,
The equilibrium of interest will be
In essence, if a disturbance occurs in the concentration of the species involved in this equilibrium reaction, the equilibrium will shift in a direction that will compensate that disturbance - think Le Chatelier's Principle.
If a strong acid is introduced in the system, which is equivalent to having an increased concentration of hydronium ions, it will react with the bicarbonate anion and form carbonic acid, a weak acid.
The equilibrium will thus shift to the left.
The fact that a strong acid is converted to a weak one will prevent to acidity of the solution to increase significantly.
Likewise, if a strong base is introduced, it will react with the carbonic acid to form the bicarbonate anion, thus reducing the potential increase in pH.
The equilibrium will shift right.
This buffer is actually used by the body to regulate blood acidity.
Check out this really cool demonstration by Professor Diane O'Dowd of how this buffer works

