What is the chemical formula for potassium sulfate?
1 Answer
The formula is
Explanation:
The name consists of two words, with the second word ending in -ate.
This tells you that the compound is a salt.
The first word is the name of the metal ion, and the second word is the name of the anion.
Step 1.
Write the symbol of the metal with its charge.
Potassium is in Group 1.
Its symbol is
Step 2.
Sulfate is a polyatomic ion.
You have memorized the formulas your instructor gave you for the polyatomic ions, haven’t you?
The formula for sulfate ion is
Step 3.
Use the crisscross method to write the formula.
Write the charge on the first ion as the subscript of the second, and the charge on the second ion as the subscript of the first.
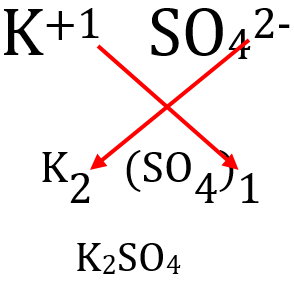
We omit subscripts of 1, and we do not include the parentheses around the polyatomic ion if its subscript is 1.
∴ The formula of potassium sulfate is

