What is the difference between diffusion and effusion?
1 Answer
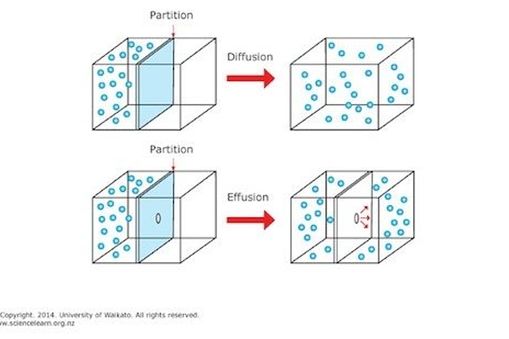
Diffusion refers to the expansion of gas to fit a larger volume; effusion refers to the passage of the gas through a small hole to fill the larger volume.
Explanation:
In diffusion the barrier between the gas and the container in which it is to expand is either non-existent or has an aperture larger than the mean free path of the gas molecules, allowing the gas to move through freely.
In effusion the barrier between the gas and the container in which it is to expand has an aperture smaller than the mean free path of the gas molecules, allowing the gas to move through less easily as only one molecule can enter at a time.
The mean free path of gas molecules refers to the average distance travelled by the gas molecule before it collides with another gas molecule. It follows that if the aperture is wider that the mean free path, the gas will diffuse freely, whereas if it is smaller, the increased chances of collision restrict the passage of the gas.