What is the difference between electron affinity and ionization energy?
1 Answer
Well, the former is a reductive process...and ionization is an oxidative process.....
Explanation:
The electron affinity is the enthalpy associated with the formation of mole of gaseous anions, from one mole of gaseous atoms, and one mole of electrons:
And ionization enthalpy is the enthalpy associated with the formation of one mole of gaseous cations, and one mole of electrons from one mole of gaseous atoms....
Data on these processes inform our view of energy levels and electronic structure....
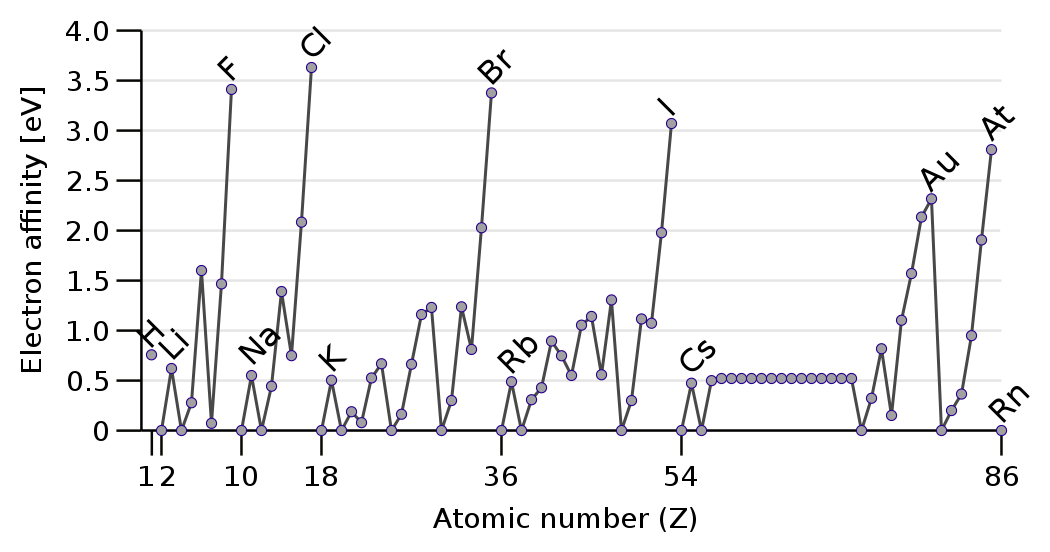
Electron affinities reasonably (why) INCREASE across a Period, from left to right as we face the Table, and decrease down a Group.
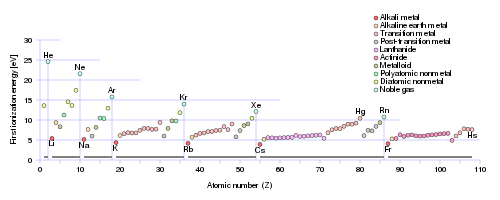
Ionization enthalpies ALSO increase across the Period, and decrease down the Group.

