What is the equation for sodium 22 undergoing electron capture?
1 Answer
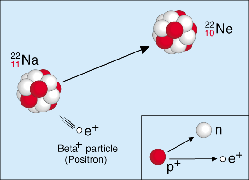
Sodium-22 undergoes electron capture according to the following nuclear equation:
Notice that the electron capture of Na-22 results in nuclear transmutation; the inner-orbital electron is captured by the nucleus, the result being the formation of a neutron from the combination of said electron with a proton from the nucleus.
This process will reduce the atomic number by 1 (
You will sometimes see the electron capture of Na-22 being described as a positron emission. The nuclear equation for that process is
Electron capture can be viewed as the equivalent process to positron decay, since both processes result in the same nuclear transmutation - the formation of