What is the esterification reaction equation of benzyl alcohol and acetic acid?
2 Answers
Explanation:
Ph stands for a phenyl group.
Here the carboxylic acid is first protonated by sulfuric acid, making the carbonyl carbon more electrophilic. The Oxygen of the alcohol then attacks this electrophilic carbon. The Hydrogen of the original alcohol oxygen will then be removed and be used to protonate the the hydroxy group of the original carboxylic acid, making a
The Oxygen protonated in the first step is then deprotonated, and forms a double bond with the central carbon to give the final product.
Essentially what you have is an acid-catalyzed dehydration; the
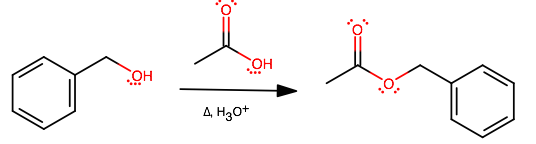
The mechanism goes as follows (the unsaid step being to protonate the carbonyl, of course, because of the high
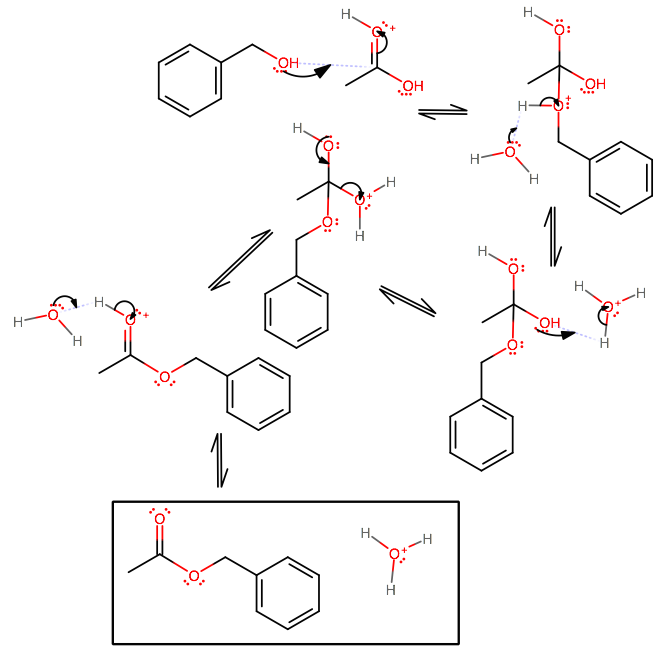
What happens is the alcohol can act as the nucleophile in this favorable circumstance (electrophilic carbonyl, hot temperatures).
Once it binds, there is a proton transfer (you will be doing this often in organic chemistry II, so know this) to either of the hydroxyl groups, and ultimately a tetrahedral collapse where the protonated hydroxyl leaves as water. See? Dehydration.
Finally, the acid catalyst is regenerated and you have your product: benzyl acetate.


