What is the first law od thermodynamics? How is this law applicable to the Earth's energy balance?
1 Answer
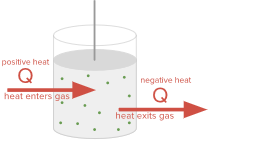
The first law of thermodynamics says that the change in internal energy of a system ΔU equals the net heat transfer into the system Q plus the work done on the system.
Explanation:
A body in space (Earth) is being radiated by a star, the Sun, and then if it remains at a constant temperature, it must radiate out as much energy as it receives.