What is the Name for PbHPO4?
1 Answer
May 8, 2015
Actually, that compound is lead (II) hydrogen phosphate, not lead (IV) hydrogen phosphate.
Notice that it contains the hydrogen phosphate anion,
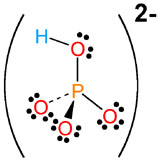
This implies that the lead cation is in a +2 oxidation state, so you're actually dealing with the lead (II) cation,
As a result, the correct name for
Lead (IV) hydrogen phosphate will have the lead cation in a +4 oxidation state, so you'd be dealing with the lead (IV) ion,
As a result, lead (IV) hydrogen phosphate would be

