Let us examine a simple acid base reaction....
#NaOH(aq) + HCl(aq) rarr NaCl(aq) +H_2O(l)#
And for the purposes of the example we assume we add a solution of sodium hydroxide FROM a burette, to a KNOWN volume of #HCl(aq)# of KNOWN concentration.
And let us consider the #pH# of such a typical strong acid/strong base titration...
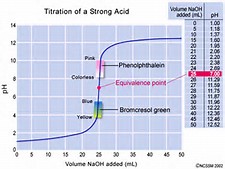
(Perhaps I could have used a BIGGER illustration...)
Now the equivalence point is CLEARLY at #pH=7.0#...and while ideally, we would like the indicator to change colour at this point, as shown in the graph, the #pH# changes sigmoidally over SIX whole pH units. And if you look at the abscissa of the #"x-axis"#, the dramatic #pH# change occurs over the addition of #0.01*mL#...and this is about #1*"drop"# of titrant...and so several indicators are feasible in the given scenario. As always with these titrations, we titrate to the POINT of colour change, NOT the colour change itself.


