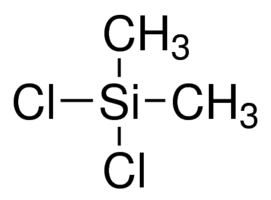
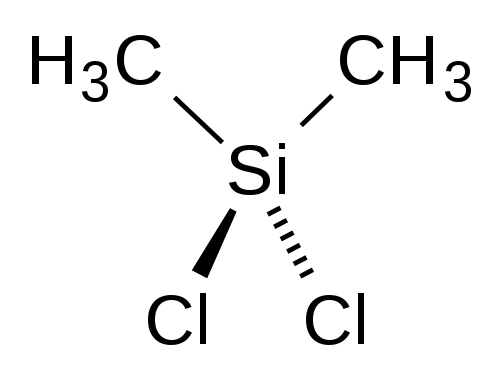
What is the Lewis Dot Structure of (CH3)2SeCl2?
What is the VSEPR Class of the molecule?
What is the VSEPR Class of the molecule?
1 Answer
The most likely structure should be
Explanation:
Using Se in this compound would mean the structure would be based upon a trigonal bipyrimidal parent structure with a see-saw geometry. There's probably not a reference in the literature for such a compound, but I'll stand corrected if it is. The most likely structure is Dimethyldichlorosilane which would be