What is the Lewis electron-dot diagram for a fluoride ion?
1 Answer
Jan 7, 2017
Fluorine is in Group 17 of the Periodic Table....................
Explanation:
And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular.
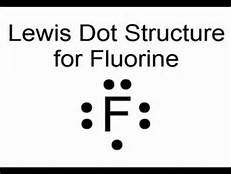
Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).

Which do you think would be bigger; fluorine atom or fluoride ion?

