What is the London dispersion force?
1 Answer
May 9, 2018
Well, this is the weakest of the intermolecular forces .... it occurs BETWEEN all ATOMS, and ALL molecules....
Explanation:
And note the distinction between
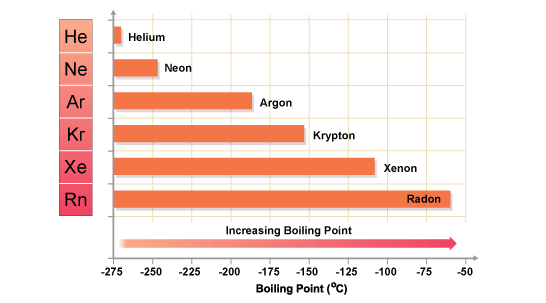
The electrons associated with all atoms can form dipoles, i.e. net accumulations of positive or negative charge .... While these are transient, i.e. very short-lived...the orientation of the dipoles can add to the interaction of the particles. And the extent of this interaction depends on the NUMBER of electrons. And thus fot the Noble Gases, for which dispersion force is the only intermolecular (interatomic) force, the resultant boiling point INCREASES down the Group for the inert gases...