What is the major difference between a cyclic hemiacetal and a cyclic acetal?
1 Answer
Nov 18, 2015
It is apparent that ketal and ketone share the same first three letters. That makes it easier to remember that:
- acetals form from aldehydes
- ketals form from ketones
That implies that:
- cyclic hemiacetals form from cyclization of aldehydes
- cyclic hemiketals form from cyclization of ketones
Let's take a look at how a cyclic hemiacetal forms.
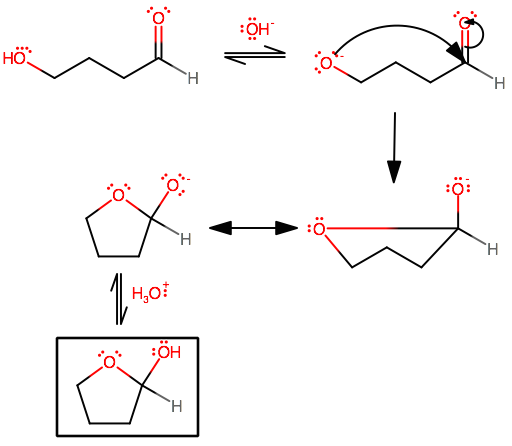
I drew the less obvious arrows, but I'll leave the proton transfers for you to practice. The formation of a cyclic hemiketal is nearly identical; simply replace the explicit hydrogen with a methyl group.

