What is the mechanism for nucleophilic substitution between butyl- iodide and NaCN?
1 Answer
This is not too complicated. The confusion here seems to lie in what the nucleophile is and what it replaces.
Nucleophilic substitution is when a nucleophile takes the place of a leaving group in a substitution reaction.
LEAVING GROUP PROPENSITY
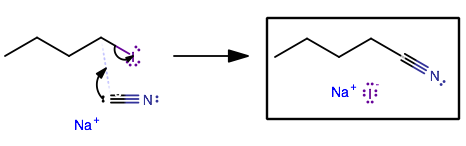
With butyl iodide and sodium cyanide, notice how big iodine is.
So, naturally we should expect iodide to be a great leaving group... that is, it has a high leaving group propensity (pKa of
WHAT'S THE NUCLEOPHILE?
And then, let's consider what the nucleophile would be. It's either iodide or cyanide, because it has to be able to attach by donating electrons to an electrophilic center. Sodium CATION doesn't have any valence electrons to donate. Neither does the butyl carboCATION.
But remember, iodide is part of butyl iodide. If we want to CHANGE the reactant to something else, there's no point in iodide attaching itself back onto butyl iodide. We want to go forwards. So cyanide must be the nucleophile.
That confirms the mechanism: either

