What is the nuclear equation for the beta decay of Ti207?
1 Answer
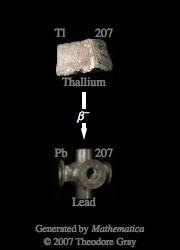
I believe you were referring to the beta decay of Thallium-207 (Tl-207), not Ti-207, since the latter, Titanium, has an atomic mass of 47.89u, not even close to 207u.
So, Tl-207 is one of the 37 isotopes of Thallium and has a half-life of 4.77 minutes, the longest half-life of naturally-occuring radioisotopes.
The nuclear equation for its beta decay is:
Tl-207
During beta decay, an electron, also called a beta-particle, is emitted from the nucleus; simultaneously, a neutron is converted into a proton in the nucleus.
This causes the atomic number to increase by 1, but leaves the atomic mass unchanged.