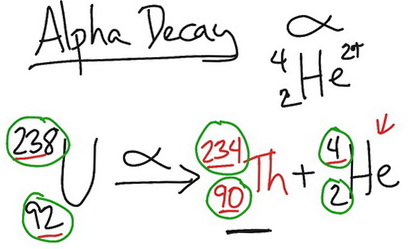What is the nuclear equation for uranium-238 after alpha radiation is emitted?
1 Answer
Aug 20, 2014
Explanation:
Uranium-238 produces thorium-234 by alpha decay.
An α-particle is a helium nucleus. It contains 2 protons and 2 neutrons, for a mass number of 4.
During α-decay, an atomic nucleus emits an alpha particle. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less.
Thus, uranium-238 decays through α-particle emission to form thorium-234 according to the equation:
Note that the sum of the subscripts (atomic numbers or charges) is the same on each side of the equation.
Also, the sum of the superscripts (masses) is the same on each side of the equation.