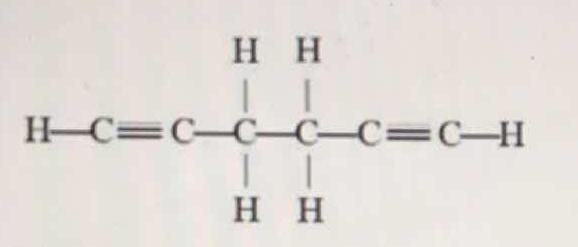
What is the number of pi bonds in the molecule below?
2 Answers
4.
Explanation:
Most of the carbon-carbon bonds in this molecule (which appears to be a 1,5-hexadiyne) are single and contain only sigma bonds. Each of the carbon-carbon triple bonds involves three pi bonds. There are two triple carbon-carbon bonds in the molecule and therefore we have four pi bonds in the molecule.
(Since the photo was a little bit shaky, here's another condensed structure formula for your reference)
http://www.chemspider.com/Chemical-Structure.62610.html
There are 4 pi bonds in the molecule shown.
Explanation:
Pi bonds are present in double or triple bonds.
A single bond contains
A double bond contains
A triple bond contains
There are 2 triple bonds in the molecule, therefore,
This post may also help you!
Check out this video on how to count sigma and pi bonds!

