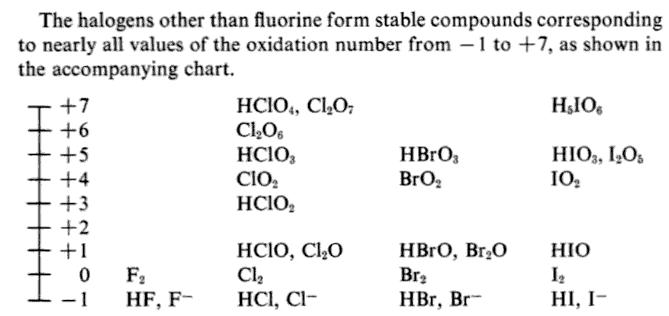
What is the oxidation number of a Group VIIA element in a compound?
1 Answer
Jul 27, 2014
The oxidation number of a Group 7 element depends on the compound it is in.
Explanation:
The most common oxidation number for Group 7 elements is -1, as in
Its oxidation number in compounds is always -1.
But the other Group 7 elements can have various oxidation states.
For example,
- +1 — in
#"NaClO"# - +3 — in
#"NaClO"_2# - +4 — in
#"ClO"_2# - +5 — in
#"KClO"_3# - +6 — in
#"Cl"_2"O"_6# - +7 — in
#"KClO"_4#