What is the oxidation number of carbon monoxide?
1 Answer
Zero.
Explanation:
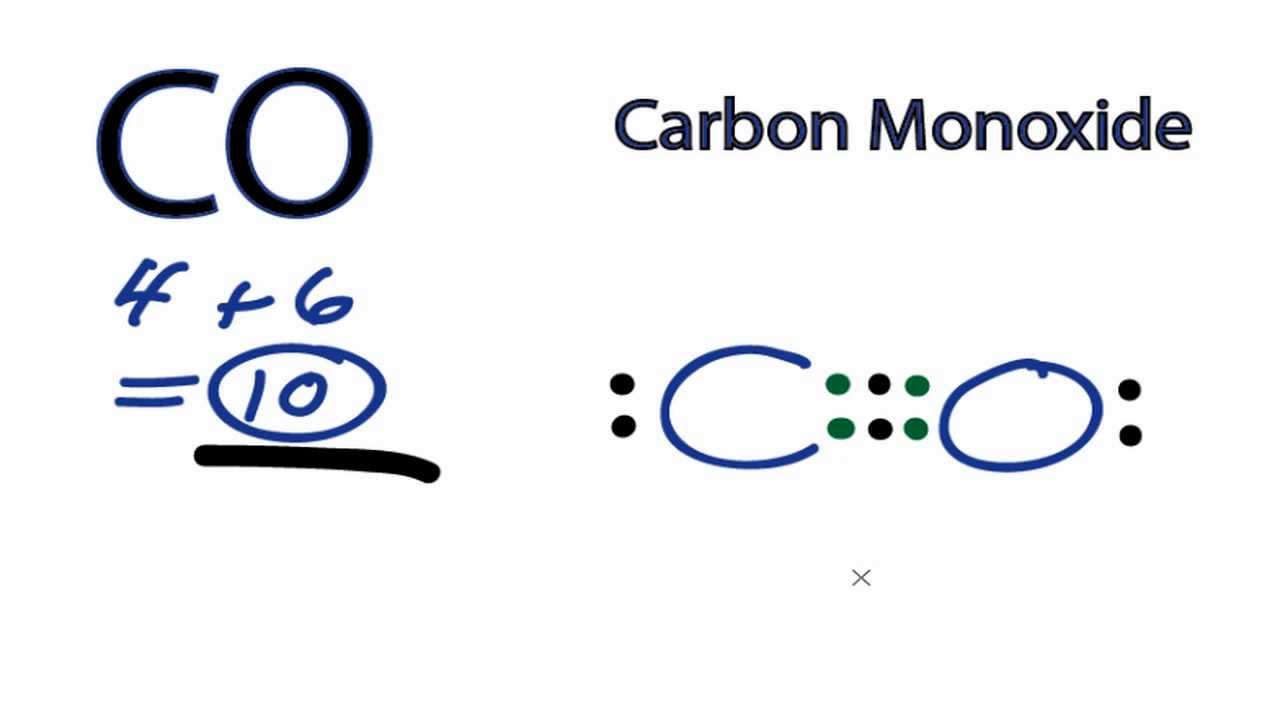
You need to consider the Lewis structure of carbon monoxide. Based on their group numbers in the periodic table, the valence electrons of
Drawing the electron dot structure of carbon monoxide,
 https://www.youtube.com/watch?v=lEpfplRxcSg
https://www.youtube.com/watch?v=lEpfplRxcSg
Notice that the structure is sharing 6 electrons (2 from
Now if you count the number of electrons per element, you would notice that there are formal charges produced in this chemical bonding.
http://www.ebi.ac.uk/chebi/advancedSearchFT.do?structureSearchMethod=similarity&chebiId=15343
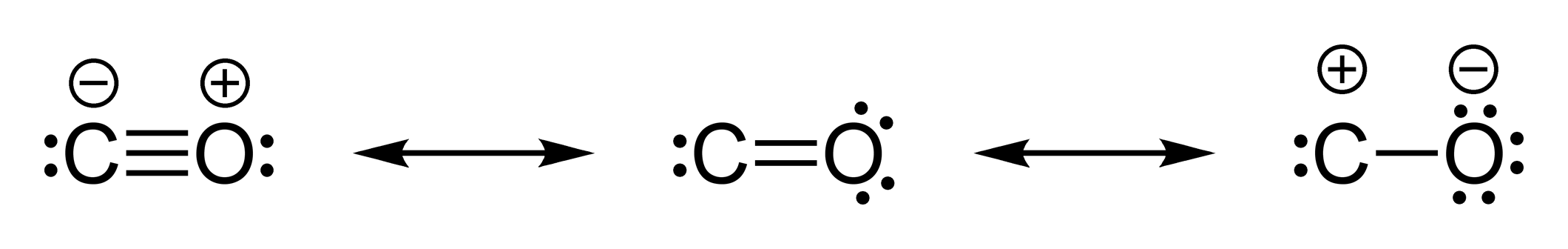
Although both atoms have a formal charge, the overall charge is still zero.
Incidentally, the
 https://commons.wikimedia.org/wiki/File:Carbon-monoxide-resonance-2Dpng
https://commons.wikimedia.org/wiki/File:Carbon-monoxide-resonance-2Dpng

