What is the oxidation state of phosphorus in PCl5?
1 Answer
Phosphorus has a
In this case, phosporus' oxidation number depends strictly on chlorine's
Here's how you'd figure out the oxidation number for both chlorine and phosphorus in
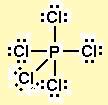
The molecule has a total of 40 valence electrons - 7 from each chlorine atom and 5 from phosphorus. Now take a look at what's happening from the perspective of bond electrons
Phosphorus contributes 1 electron, all of them drawn in red in the above image, to each bond it forms with chlorine. Now, oxidation numbers are assigned with one simple concept in mind: the more electronegative atom of the two takes both bonding electrons.
In this case, chlorine is more electronegative than phosphorus, which means that each chlorine atom will take the electron phosphorus used for the bond.
As a result, each chlorine atom will have one extra electron, which means an O.N. of (-1); at the same time, phosporus now has five less electrons that it had before bonding with the 5 chlorine atoms

