What is the product of the aldol condensation between cyclopentanone and 4-methylbenzaldehyde?
1 Answer
The product is probably (
Explanation:
The aldol condensation involves attack of an enolate ion on the base of a carbonyl compound to form a β-hydroxycarbonyl compound (an aldol).
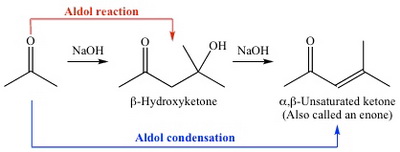
(from www.chem.ucla.edu)
The aldol can then dehydrate to form an α,β-unsaturated carbonyl compound.
The process requires an active methylene adjacent to a carbonyl group.
If there are two active methylenes, the condensation can occur on each side of the carbonyl group.
For example, benzaldehyde with acetone gives dibenzalacetone.

By analogy, I predict that 4-methylbenzaldehyde with cyclopentanone will form
(
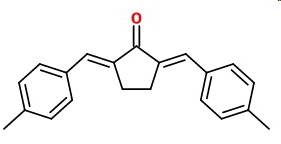
The

