What is the relative size of the radius of a positive ion to its neutral atom?
1 Answer
Jan 22, 2017
Well, in ionizing an atom, you are knocking off 1 or more valence electrons........
Explanation:
And given the definition of atomic radius, the radius of an isolated atom is defined by its electronic radius, the ionic radius should be vastly reduced with respect to the atomic radius.
But as chemists, as physical scientists, we should take a look at the numbers, i.e. consider quantitative data.
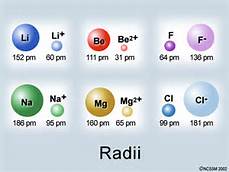
The ionic radius of the metal ion is dwarfed by the radius of the parent metal ion. On the other hand, the ionic radius of the non-metal, which is REDUCED to give the ion, dwarfs that of the parent non-metal atom. Measurements are given in
Is this consistent with our argument?

