What is the sum of the oxidation numbers in a polyatomic ion?
1 Answer
The sum of the oxidation numbers in a polyatomic ion is equal to the net charge of that respective ion.
If a polyatomic ions has a -1 net charge, then the sum of its elements' oxidation numbers must be -1; likewise, if a polyatomic ions has a +2 net charge, then the sum must be +2, and so on.
Here are some examples of this principle
- Ammonium,
#NH_4^(+)#
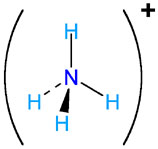
The net charge of the ion is +1, which means that the oxidation numbers of each of the four hydrogen atoms and that of nitrogen must add up to +1.
Since hydrogen has a +1 oxidation numbr (ON), nitrogen will have a -3 ON to accomodate for the charge on the ammonium ion.
- Sulfate,
#SO_4^(2-)#
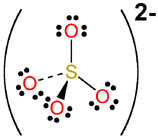
This time, the ion has a -2 net charge. Because oxygen has an ON of -2, the ON of sulfur will be +6.
- Phosphate,
#PO_4^(3-)#
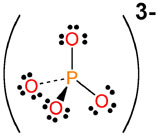
Since the net charge on the ion is -3, the oxidation numbers of all the atoms that make up this polyatomic ion must add up to -3.
Each oxygen atom has an ON of -2, which implies that the phophorus atom will have an ON of +5.

