What is the titration curve of glycine?
1 Answer
The titration curve for glycine looks like the titration curve for a weak diprotic acid.
Explanation:
Below is a typical curve for the titration of glycine with NaOH.
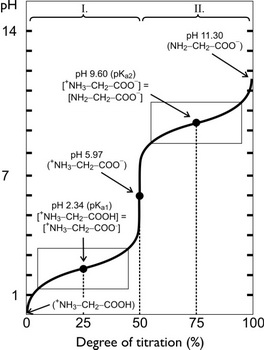
(from elte.prompt.hu)
Although we often write glycine as NH₂COOH, it is really a zwitterion,
The fully protonated form of glycine is
The protonated form of glycine ionizes in two steps:
Step 1 is the loss of
Step 2 is the loss of
The first equivalence point, at 50 % titration, is at
Halfway between 0 % and 50 % titration (i.e. at 25 %)
The second equivalence point, at 100 % titration, is at
Halfway between 50 % and 100 % (i.e. at 75 %),
At 50 % titration, the glycine exists as a zwitterion.
This is the isoelectric point
At this point,
For glycine,
Each amino acid has a characteristic set of
Thus, you can use a titration curve to identify an unknown amino acid.

