What is the VSEPR shape of the molecule #PF_3#?
1 Answer
May 8, 2014
The VSEPR shape of the molecule
Explanation:
We can use VESPR theory to predict a trigonal pyrimidal shape for the molecule
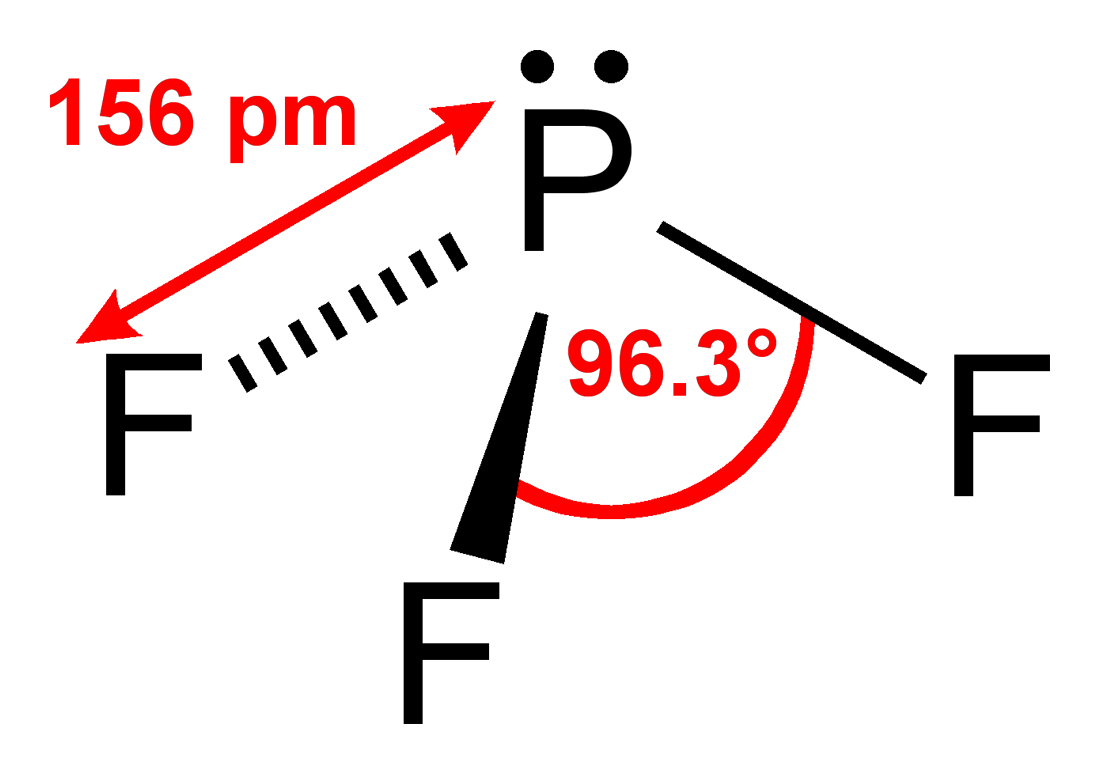
VESPR stands for valence shell electron pair repulsion. This theory basically says that bonding and non-bonding electron pairs of the central atom in a molecule will repel (push away from) each other in three dimensional space and this gives the molecules their shape.
We can use the following notations when examining a Lewis structure of a molecule.
A = central atom
X = peripheral atoms
E = non-bonding electron pairs of the central atom


