What is the Zeroth law of thermodynamics?
1 Answer
Oct 27, 2014
The zeroth law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third, then all three are in thermal equilibrium with each other.
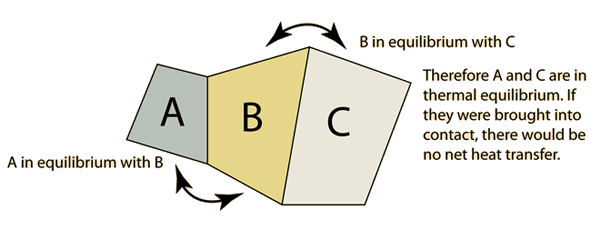
Taking an example:
If A and C are in thermal equilibrium with B, then A is in thermal equilibrium with C.
Basically, it would mean that all three: A, B and C are at the same temperature.
The Zeroth Law is so named because it logically precedes the First and Second Laws of Thermodynamics.
