What Is Z component of orbital angular momentum? How can we find the Z component? What is its importance? What does it resemble?
1 Answer
You seem to be referring to
Practically speaking, for general chemistry, you can simply use the value of
#bb(m_l = {-l,-l+1, . . . , 0, . . . , l - 1, l})#
For instance, if
#m_l = {-2,-1,0,+1,+2}#
That means five
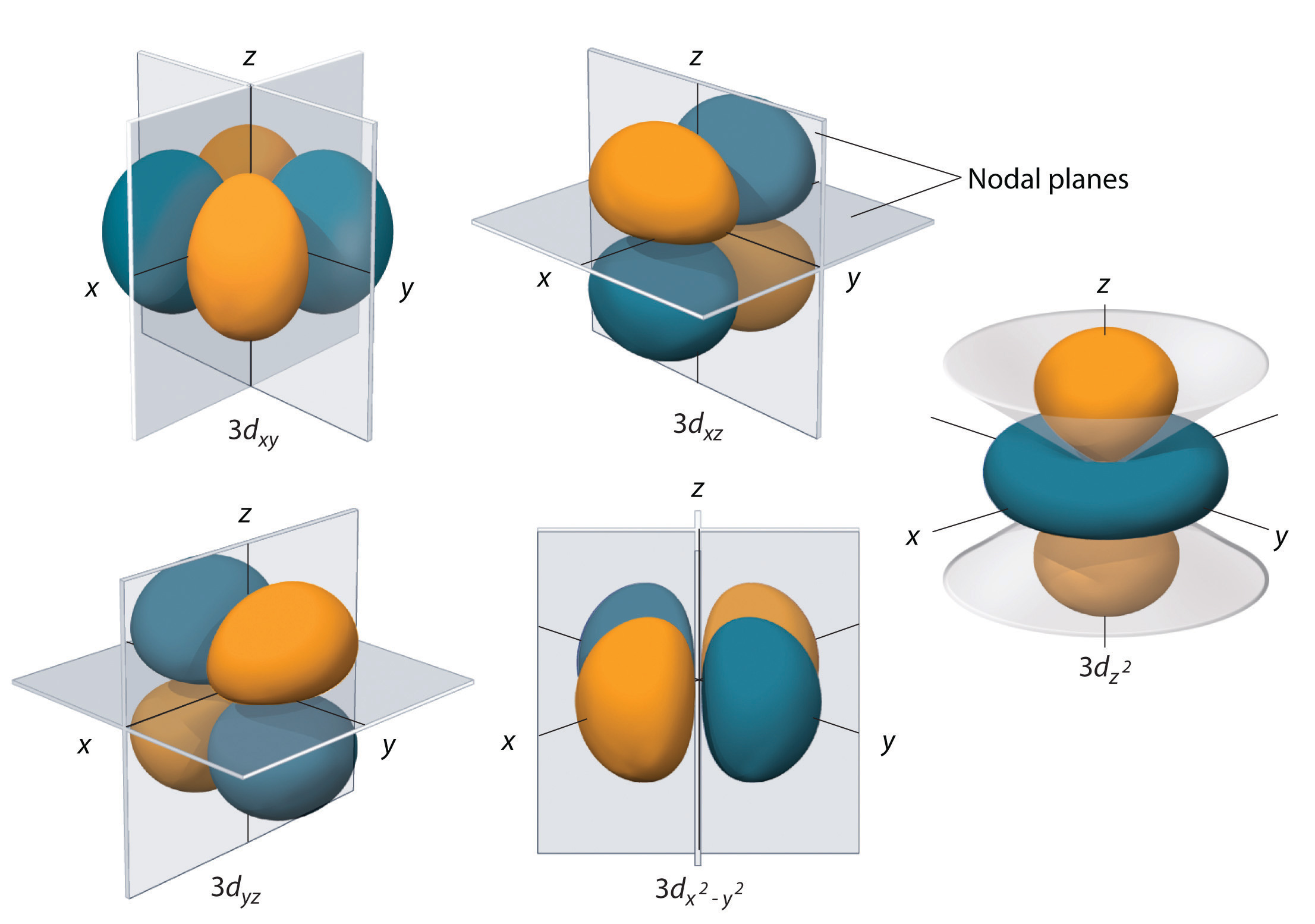
RELATION TO THE Z-COMPONENT OF THE TOTAL ORBITAL ANGULAR MOMENTUM
Recall that the Schrodinger equation is typically written as
Well, it turns out that
#psi_(nlm_l)(r,theta,phi) = R_(nl)(r)Y_(l)^(m_l)(theta,phi)#
where
Traditionally,
This eigenvalue corresponds to the operator for
What we just said can be expressed as:
#stackrel("Operator")overbrace(hatL_z)" "stackrel("Angular")stackrel(" Component")stackrel("of Wave Function")overbrace(Y_(l)^(m_l)(theta,phi)) = stackrel("Eigenvalue")overbrace(m_lℏ)" "stackrel("Angular")stackrel(" Component")stackrel("of Wave Function")overbrace(Y_(l)^(m_l)(theta,phi))#
If
PHYSICS PERSPECTIVE
Visually, in the presence of a magnetic field in the
This is the event described by
For instance, when
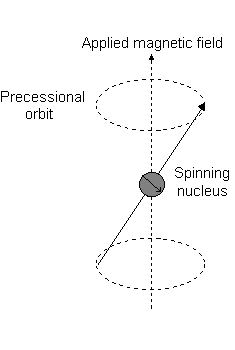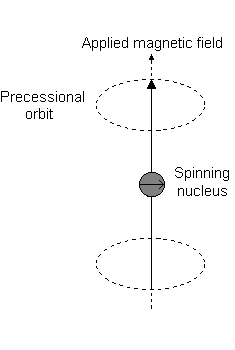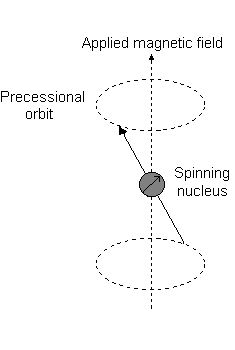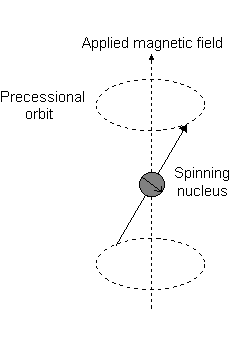
And each
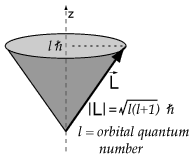
For instance:
- An
#m_l# of#1# corresponds to the upper half of the#2p_z# orbital. - An
#m_l# of#0# is the dot at the origin. - An
#m_l# of#-1# corresponds to the lower half.
CHEMISTRY PERSPECTIVE
From a practical point of view, what we really care about is how to use
- The number of
#m_l# values tells you the number of orbitals in a subshell. - The range of
#m_l# is based on the chosen#l# .
For example, since
#m_l = {-2,-1,0,+1,+2}#
That means five


