What isomeric Lewis structure of #CN_2H_2# has no formally charged atoms?
1 Answer
Both H-N=C=N-H and H₂N-C≡N have no formally charged atoms.
"They" don't tell you the connectivity of the atoms, so we have to consider all possibilities.
Here's one way to figure out the structures:
1. Write all possible connections for the non-hydrogen atoms.
N-C-N and C-N-N
2. Add the H atoms.
There are 5 reasonable combinations:
H₂N-C-N or H-N-C-N-H or H₂C-N-N or H-C-N-N-H or C-N-NH₂
You will find that structures with H on the central atom are impossible.
3. Count
4. Calculate
where
5. Draw new structures.
This time insert the double and triple bonds in all possible combinations.
6. Add valence electrons to give each atom an octet.
7. Calculate the formal charge on each atom.
We end up with 11 possibilities.
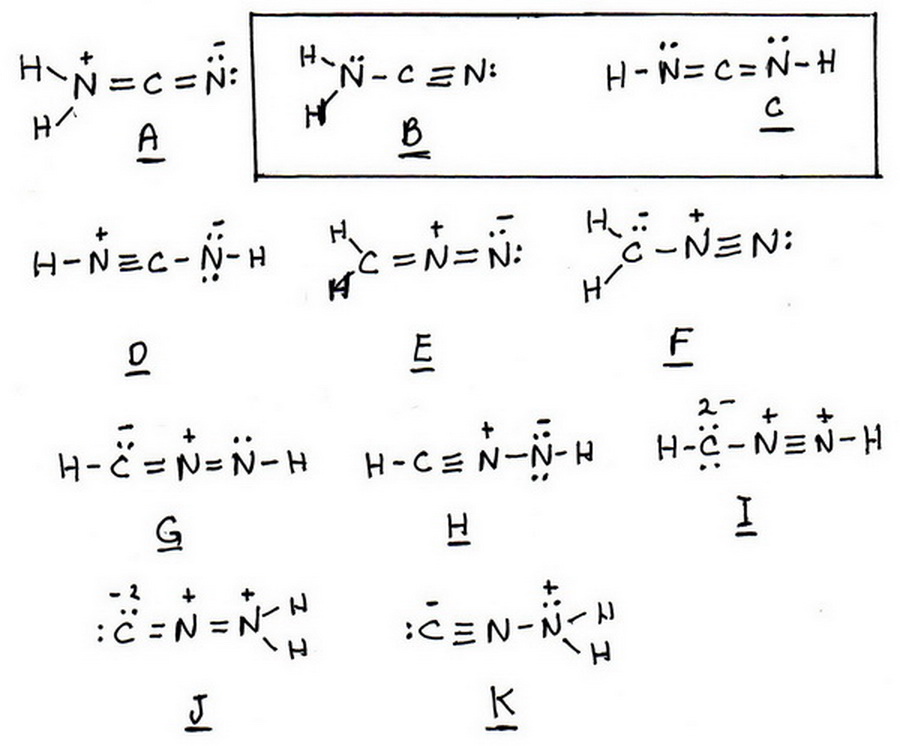
8 Identify the structures that have no formal charges.
The only structures that have no formal charges are B and C.
These are two different structures of the compound called cyanamide.

